FDA Dietary Supplements
&
Good Manufacturing Practice(GMP)

Certification of Dietary Supplements
FDA & NSF Involvement
Q&A
Q1: Who ensures the safety dietary supplements In the US?
Supplement manufacturers are responsible by law to ensure that their products are safe before being marketed. In addition, manufacturers are responsible for determining the accuracy and truth of label claims. However, the US Food and Drug Administration (FDA) can take action against:
- any unsafe dietary supplement product that reaches the market
- as well as any false or misleading claims

Q2: How does regulation of supplements differ from that of prescription or over-the-counter drugs?
Before sales and distribution, drugs must undergo clinical studies to determine their effectiveness, safety, possible interactions with other substances and appropriate dosages. FDA then reviews this data and determines whether to authorize use of the drugs. Dietary Supplements fall under the general category of food products. Unless they contain a new ingredient, dietary supplements are not tested or authorized for use prior to being marketed. Please note that the FDA has oversight over these products and can limit the type of ingredients used in product formulations and take action when false or misleading label claims are made.
Q3: How many different levels of certification are there for dietary supplements?
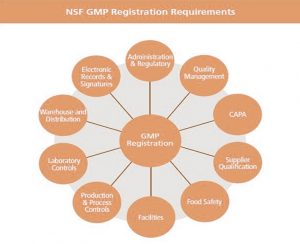
Currently two. For the first level, NSF International provides GMP (Good Manufacturing Practice) registration for companies. This requires a manufacturer to bring the NSF organization into the firm’s production sites, to validate that the manufacturer is following the GMP’s defined for their respective industry.
At each facility, there are customarily two NSF auditor visits a year. This certification is not a product certification, but ensures that companies follow best-practice procedures for:
- production and process control systems,
- personnel,
- physical plant and grounds,
- equipment and utensils,
- storage and distribution,
- etc.
This GMP certification ensures a product has the identity, strength, composition, quality and purity that appears on its label. These GMP requirements are listed in Section 8 of NSF/ANSI 173. This is the only accredited American National Standard in the dietary supplement industry developed in accordance with the U.S. FDA’s 21 CFR part 111.
The second level involves certification in accordance with NSF/ANSI 455, which is an expanded certification and transition from the original NSF/ANSI 173 (section 8) GMP. (Please note that standalone warehouse and distribution facilities will remain under NSF/ANSI 173 GMP for the foreseeable future.)
The newer NSF/ANSI 455 standards was issued in January 2019, by the Global Retailer and Manufacturer Alliance (GRMA) . NSF/ANSI 455 is a set of consensus-based Good Manufacturing Practices (GMP) requirements for manufacturers of:
- dietary supplements (NSF/ANSI 455-2),
- cosmetics and personal care products (NSF/ANSI 455-3) and
- over-the-counter drugs (NSF/ANSI 455-4 ).
There are major changes in NSF/ANSI 455, in comparison to the original:
- Approved document contents/format
- Audit Grade Rules
- Post Audit Deadlines and Corrective Action Requests (CAR’s)
Approved Document Contents/Format
The new NSF/ANSI 455-2 GMP document set can be seen to the right. The original NSF GMP document standard may be seen to the right above.
The new standard maintains only seven document sets, whereas the original standard maintained ten.
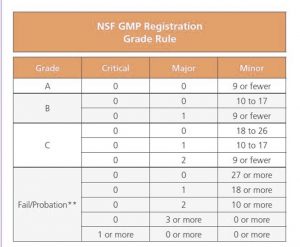
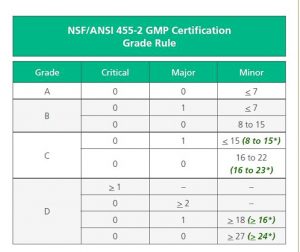
Audit Grade Rules
The Audit Grade Rules have changed significantly from the original NSF GMP Registration, to the NSF/ANSI 455-2 standard. Major changes include the following;
- A reduction in minor infractions from 9 to 7, in order to obtain an ‘A’ grade.
- A reduction in minor infractions from a maximum of 17 to a maximum of 15 to maintain a ‘B’ grade.
- A removal of the two major infraction option from the ‘C’ grade.
- A removal of three or more major infractions as an option for a ‘D’ grade.
*
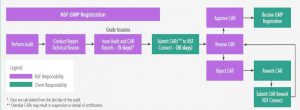
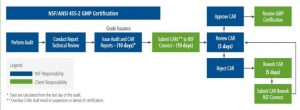
Post Audit Deadlines and Corrective Action Requests (CAR’s)
A very major change for Corrective Action Requests (CAR’s) has been implemented as the result of the transition from NSF GMP to NSF/ANSI 455-2 .
Specifically, the number of days allowed to submit Corrective Action Requests (CAR’s) back to NSF has dropped dramatically from 30 to 10.
Deadline to Move from NSF GMP to NSF/ANSI 455-2 GMP
.NSF started performing audits consistent with NSF/ANSI 455-2 GMP Certification in ovember 2019. The current timetable for transition is as follows:
- Through December 31, 2021 – Clients can select audits to NSF GMP Registration or NSF/ANSI 455-2 GMP Certification.
- After December 31, 2021 – NSF GMP Registration audits will be retired and replaced with NSF/ANSI 455-2 GMP Certification.
- Standalone Warehouse & Distribution facilities will remain under the original NSF GMP Registration program for the foreseeable future.



We WILL deliver the solution that you need !
As a first step, we will be delighted to answer any and all of your questions !